This Chemistry quiz is called 'Atomic Structure 3' and it has been written by teachers to help you if you are studying the subject at high school. Playing educational quizzes is a user-friendly way to learn if you are in the 9th or 10th grade - aged 14 to 16.
It costs only $12.50 per month to play this quiz and over 3,500 others that help you with your school work. You can subscribe on the page at Join Us
The study of atomic structure forms a large part of high school Chemistry. This quiz looks not only at the numbers and arrangement of protons, neutrons and electrons in atoms, their atomic numbers and their atomic mass, but also at the numbers of electrons in the different shells of atoms and how the electrons in an atom’s outer shell are affected when ions are formed.
[readmore]
One of the biggest hurdles to understanding chemistry is the idea of scale. With atomic structure, we are dealing with processes and objects that we cannot see directly which is why it was not until well into the 20th century that protons and neutrons were identified. Our knowledge of what lies inside an atom has been discovered by using indirect observations, for example, the scattering of alpha particles as they passed through a thin piece of gold foil demonstrated that the mass of an atom was concentrated into a very small area at the center - the nucleus.
The first of the sub-atomic particles that were identified was the electron. Experiments using 'cathode rays' in 1897 by J.J. Thompson showed that they were the same no matter what element they came from. He guessed that they were some kind of fundamental particle that was a structural part of all atoms. They had a negative charge and in 1904 he suggested that an atom consisted of these 'corpuscles' whizzing round in a 'sea' of positive charge. We now know them as electrons which do move round in atoms but they do so in well defined areas known as 'shells' or 'energy levels'. They remain part of the atom because of the attraction of the nucleus. We also now appreciate that each of the 'shells' contains a specific maximum number of electrons. You only need to know how to work out the arrangement of electrons in each of the first 20 elements and their ions for high school.
For the exam, you need to be able to recognize elements from their electron arrangements and state what happens to electrons and the electron structure of atoms when they bond with other atoms, including the formation of ions.
[/readmore]
|
1.
|
An atom of which element has 11 electrons? 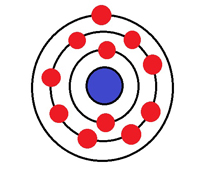
|
|
| [ ] |
Potassium |
| [ ] |
Sodium |
| [ ] |
Magnesium |
| [ ] |
Neon |
|
|
|
2.
|
Which element has 2 electrons, 2 neutrons and 2 protons? 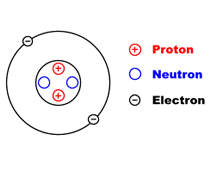
|
|
| [ ] |
Fluorine |
| [ ] |
Sodium |
| [ ] |
Helium |
| [ ] |
Neon |
|
|
|
3.
|
An atom contains 9 electrons. How many neutrons are there in the nucleus of this atom? 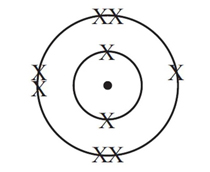
|
|
| [ ] |
10 |
| [ ] |
9 |
| [ ] |
19 |
| [ ] |
11 |
|
|
|
4.
|
An atom has 7 electrons - 2 in its inner shell and 5 in its outer shell. What group of the periodic table is this element found in? 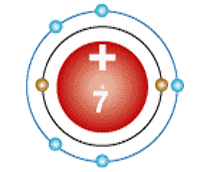
|
|
|
|
|
5.
|
An atom has 2 electrons, 2 neutrons and 2 protons. What is the atomic number of this atom? 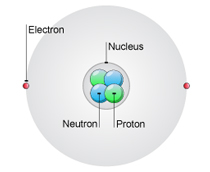
|
|
|
|
|
6.
|
A fluorine atom has 9 electrons - 7 in its outer shell and 2 in its inner shell. How many electrons would be in the outer shell if it were to become a fluoride ion? 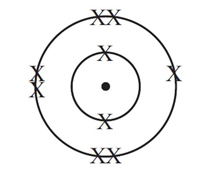
|
|
|
|
|
7.
|
How many electrons are in the outer shell of a nitride 3- ion? 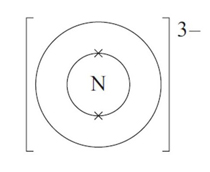
|
|
|
|
|
8.
|
An element has 2 electrons in its inner shell, 8 in its next shell and 1 in its outer shell. What type of ion would it form? 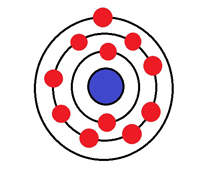
|
|
| [ ] |
1- |
| [ ] |
1+ |
| [ ] |
2+ |
| [ ] |
3- |
|
|
|
9.
|
How many electrons are in the first shell of a hydrogen atom? 
|
|
|
|
|
10.
|
How many electrons are in the first shell of a carbon atom? 
|
|
|
|
|
1.
|
An atom of which element has 11 electrons? 
|
|
| [ ] |
Potassium |
| [x] |
Sodium |
| [ ] |
Magnesium |
| [ ] |
Neon |
|
|
|
2.
|
Which element has 2 electrons, 2 neutrons and 2 protons? 
|
|
| [ ] |
Fluorine |
| [ ] |
Sodium |
| [x] |
Helium |
| [ ] |
Neon |
|
|
|
3.
|
An atom contains 9 electrons. How many neutrons are there in the nucleus of this atom? 
|
|
| [x] |
10 |
| [ ] |
9 |
| [ ] |
19 |
| [ ] |
11 |
|
|
|
4.
|
An atom has 7 electrons - 2 in its inner shell and 5 in its outer shell. What group of the periodic table is this element found in? 
|
|
|
|
|
5.
|
An atom has 2 electrons, 2 neutrons and 2 protons. What is the atomic number of this atom? 
|
|
|
|
|
6.
|
A fluorine atom has 9 electrons - 7 in its outer shell and 2 in its inner shell. How many electrons would be in the outer shell if it were to become a fluoride ion? 
|
|
|
|
|
7.
|
How many electrons are in the outer shell of a nitride 3- ion? 
|
|
|
|
|
8.
|
An element has 2 electrons in its inner shell, 8 in its next shell and 1 in its outer shell. What type of ion would it form? 
|
|
| [ ] |
1- |
| [x] |
1+ |
| [ ] |
2+ |
| [ ] |
3- |
|
|
|
9.
|
How many electrons are in the first shell of a hydrogen atom? 
|
|
|
|
|
10.
|
How many electrons are in the first shell of a carbon atom? 
|
|
|
|










