This Chemistry quiz is called 'Bonding' and it has been written by teachers to help you if you are studying the subject at high school. Playing educational quizzes is a user-friendly way to learn if you are in the 9th or 10th grade - aged 14 to 16.
It costs only $12.50 per month to play this quiz and over 3,500 others that help you with your school work. You can subscribe on the page at Join Us
Molecules are formed when atoms bond together. Chemical bonding is hugely important in chemistry and, unsurprisingly, it forms a large part of the high school Chemistry syllabus. This quiz looks at some of the types of bonding – covalent, ionic and metallic - which occur between different elements.
[readmore]
A chemical bond is an attraction between atoms. Everything in nature wants to be stable. For atoms, this means having a full outer shell of electrons. To do this, atoms need to have the electron structure of one of the unreactive noble gasses. These are the elements in the group at the extreme right hand end of the most commonly used periodic table. There are different ways of numbering the groups of the periodic table. Most UK exam boards still follow the convention that the number of electrons in the outer shell = the number of the group. If you study chemistry to higher levels, you will find that this is no longer the case other than for groups 1 and 2, so most schools teach that the noble gasses are in group 8, but a few call it group 0 or group 18.
To achieve this, atoms give away electrons to other atoms; some will take electrons from other atoms and some will share their outer electrons with other atoms. How atoms achieve states of stability determines which types of bonding they undertake; whether it be covalent or ionic. There are other types of bonding too but you will only need to learn about covalent, ionic and metallic bonding for your high school exams.
The type of bonding also determines the structure of a material. Covalently bonded compounds usually form individual molecules but there are some covalent substances, like diamond and graphite, which have giant structures. Ionic compounds are never found as molecules, they are always giant lattices that sometimes form very beautiful crystals. In the right circumstances, crystals can be enormous - there are people who make a living from finding and selling these.
Bonding also gives rise to the properties of substances like hardness, solubility in water, melting point and boiling point but it is not always clear-cut - there are some molecules that behave partly like ionic compounds and vise versa. Understanding bonding can help you to understand the behavior of chemicals as well as helping you to predict and explain the behavior of unknown substances.
[/readmore]
|
1.
|
What type of structure does the carbon in diamond form? 
|
|
| [ ] |
Giant structure |
| [ ] |
Giant covalent structure |
| [ ] |
Covalent bond |
| [ ] |
Ionic bond |
|
|
|
2.
|
The type of bonding that holds the atoms together in water is... 
|
|
| [ ] |
covalent |
| [ ] |
hydrogen |
| [ ] |
ionic |
| [ ] |
metallic |
|
|
|
3.
|
The type of bonding that holds the atoms together in salt is... 
|
|
| [ ] |
covalent |
| [ ] |
hydrogen |
| [ ] |
ionic |
| [ ] |
metallic |
|
|
|
4.
|
What type of bonding is present in gold? 
|
|
| [ ] |
Covalent |
| [ ] |
Hydrogen |
| [ ] |
Ionic |
| [ ] |
Metallic |
|
|
|
5.
|
What type of bonding occurs when electrons are shared between the atoms in a molecule? 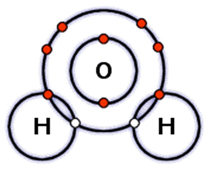
|
|
| [ ] |
Covalent |
| [ ] |
Hydrogen |
| [ ] |
Ionic |
| [ ] |
Metallic |
|
|
|
6.
|
What type of bonding occurs when electrons are transferred from the outer shell of one atom to the outer shell of another? 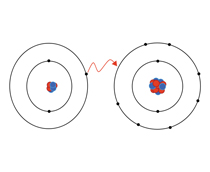
|
|
| [ ] |
Covalent |
| [ ] |
Hydrogen |
| [ ] |
Ionic |
| [ ] |
Metallic |
|
|
|
7.
|
In an ammonia molecule hydrogen and nitrogen atoms share electrons. What kind of bonding is this? 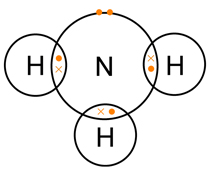
|
|
| [ ] |
Covalent |
| [ ] |
Hydrogen |
| [ ] |
Ionic |
| [ ] |
Metallic |
|
|
|
8.
|
What type of bonding has 'free' electrons? 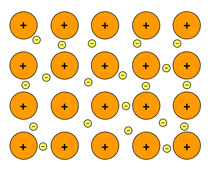
|
|
| [ ] |
Covalent |
| [ ] |
Hydrogen |
| [ ] |
Ionic |
| [ ] |
Metallic |
|
|
|
9.
|
Glucose molecules are formed from atoms of carbon, hydrogen and oxygen. What type of bonding is present in a glucose molecule? 
|
|
| [ ] |
Covalent |
| [ ] |
Hydrogen |
| [ ] |
Ionic |
| [ ] |
Metallic |
|
|
|
10.
|
Graphite is a substance made of carbon atoms only, as is diamond. However, the two substances are very different. Why? 
|
|
| [ ] |
The atoms in diamond are arranged in layers and the atoms in graphite are arranged in a giant covalent structure |
| [ ] |
The atoms in diamond are arranged in a giant covalent lattice and the atoms in graphite are arranged in layers |
| [ ] |
The atoms in diamond are arranged in a giant covalent structure and the atoms in graphite are arranged at random |
| [ ] |
The atoms in diamond are arranged at random and the atoms in graphite are arranged in layers |
|
|
|
1.
|
What type of structure does the carbon in diamond form? 
|
|
| [ ] |
Giant structure |
| [x] |
Giant covalent structure |
| [ ] |
Covalent bond |
| [ ] |
Ionic bond |
|
|
|
2.
|
The type of bonding that holds the atoms together in water is... 
|
|
| [x] |
covalent |
| [ ] |
hydrogen |
| [ ] |
ionic |
| [ ] |
metallic |
|
|
|
3.
|
The type of bonding that holds the atoms together in salt is... 
|
|
| [ ] |
covalent |
| [ ] |
hydrogen |
| [x] |
ionic |
| [ ] |
metallic |
|
|
|
4.
|
What type of bonding is present in gold? 
|
|
| [ ] |
Covalent |
| [ ] |
Hydrogen |
| [ ] |
Ionic |
| [x] |
Metallic |
|
|
|
5.
|
What type of bonding occurs when electrons are shared between the atoms in a molecule? 
|
|
| [x] |
Covalent |
| [ ] |
Hydrogen |
| [ ] |
Ionic |
| [ ] |
Metallic |
|
|
|
6.
|
What type of bonding occurs when electrons are transferred from the outer shell of one atom to the outer shell of another? 
|
|
| [ ] |
Covalent |
| [ ] |
Hydrogen |
| [x] |
Ionic |
| [ ] |
Metallic |
|
|
|
7.
|
In an ammonia molecule hydrogen and nitrogen atoms share electrons. What kind of bonding is this? 
|
|
| [x] |
Covalent |
| [ ] |
Hydrogen |
| [ ] |
Ionic |
| [ ] |
Metallic |
|
|
|
8.
|
What type of bonding has 'free' electrons? 
|
|
| [ ] |
Covalent |
| [ ] |
Hydrogen |
| [ ] |
Ionic |
| [x] |
Metallic |
|
|
|
9.
|
Glucose molecules are formed from atoms of carbon, hydrogen and oxygen. What type of bonding is present in a glucose molecule? 
|
|
| [x] |
Covalent |
| [ ] |
Hydrogen |
| [ ] |
Ionic |
| [ ] |
Metallic |
|
|
|
10.
|
Graphite is a substance made of carbon atoms only, as is diamond. However, the two substances are very different. Why? 
|
|
| [ ] |
The atoms in diamond are arranged in layers and the atoms in graphite are arranged in a giant covalent structure |
| [x] |
The atoms in diamond are arranged in a giant covalent lattice and the atoms in graphite are arranged in layers |
| [ ] |
The atoms in diamond are arranged in a giant covalent structure and the atoms in graphite are arranged at random |
| [ ] |
The atoms in diamond are arranged at random and the atoms in graphite are arranged in layers |
|
|










