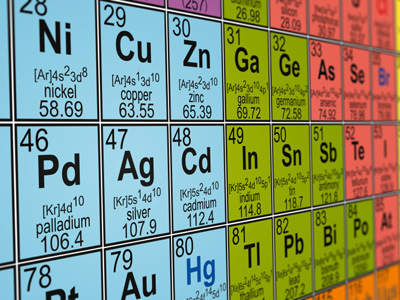
A periodic table is the means by which all chemical elements are organized based on their atomic number, electron configurations and their recurring chemical properties.
There are four common elements that include earth, air, water and fire. An element is any substance that cannot be broken into two or more simpler substances. Besides the common elements, we also have chemical elements.
A chemical element is a substance that consists of a single type of atom. Chemical elements are divided into metals, metalloids and non-metals.
Examples include carbon, nitrogen, oxygen, silicon, aluminum, iron, gold, lead, mercury, copper and arsenic. The lightest chemical elements include helium, hydrogen, lithium and beryllium. Any two atoms that have the same number of protons in their respective nucleus belong to the same chemical element.
Elements listed on the period table are listed in order of increasing atomic number. The periodic table is comprised of a grid of elements laid out in 18 columns and 7 rows. Below that grid is a double row of additional elements. The periodic table only contains chemical elements. It does not contain mixtures, compounds or subatomic particles (particles that are smaller than an atom).
The rows found in the periodic table are referred to as “periods” while the columns are known as “groups.”
Dmitri Mendeleev has been credited with publication of the periodic table in 1869. The table at that time was developed with the then-known elements. He, however, left gaps for un-known elements.
The number of protons in the nucleus of an atom is known as the atomic number. As every element has a different number of protons, they each own their own atomic number.
The elements listed on the periodic table range from atomic number 1 (which is hydrogen) to 118 (which is an unknown element). These elements have been discovered or synthesized. However, elements 113, 115, 117 and 118 have yet to be confirmed elements. As of the year 2013, the periodic table had 114 confirmed elements. Of those, 98 occur naturally while 16 elements only occur when synthesized in a laboratory.
The electron configuration is the distribution of electrons found in an atom or molecule. They describe electrons as each move independently in an orbital fashion. In order to understand the structure of the periodic table, it is important to have some knowledge as to the electron configuration of different atoms.
Chemical properties are any material that is discovered through a chemical reaction. In other words, you cannot determine the chemical property of a substance merely by looking at it or touching it. Rather a catalyst, which itself is a chemical property, needs to be used to breakdown the chemical to learn its make up.