
Atomic Structure 1
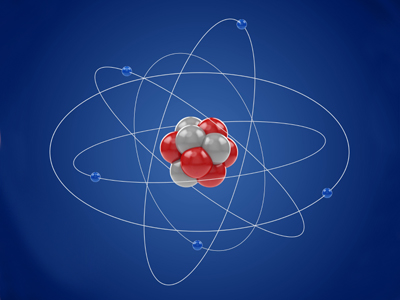
Atoms are tiny building blocks made of protons, neutrons and electrons. This GCSE Chemistry quiz helps you read atomic numbers, work out masses, and understand electron arrangement.
The atomic number is also called the proton number because it tells you the number of protons in the nucleus. For the GCSE, you need to know that elements are neutral atoms. In a neutral atom, the number of electrons orbiting the nucleus is the same as the number of protons within the nucleus. If you are not sure why, ask your science teacher to explain
The mass number shown in the Periodic Table is an average of the mass of the isotopes
Ready for more?
not all...
quizzers. Try to win a coveted spot on our Hall of Fame Page.






