Ask the AI Tutor
Need help with Periodic Table 3? Ask our AI Tutor!
AI Tutor - Lucy
Connecting with Tutor...
Please wait while we establish connection

Periodic Table 3
Groups and periods reveal repeating patterns. This GCSE Chemistry quiz explains why elements in the same group behave alike, and how electron shells help you predict reactions.
1 .
Which group of the periodic table is this?
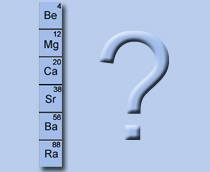
1
2
7
8
They all have 2 outer electrons available for ionic bonding
2 .
What name is given to this group of the periodic table?

Alkali earth metals
Transition metals
Noble gases
Halogens
It is also referred to as group 8 or group 0, which was its original group number when it was added to Mendeleev's periodic table
3 .
What do all the elements in this group have in common?

They all have a full outer shell of electrons
They all form 2+ ions
They all form 2- ions
They all have 1 electron in their outer electron shell
Group 1 = 1 outer electron
4 .
What do all the elements in this group NOT have in common?

They all have 6 electrons in their outer shell
They are all poisonous
They all gain one electron to form a 1- ion
They are all coloured
They have 7 electrons in their outer shell. Group 7 = 7 electrons. The other three answers are all properties of the halogens that you are expected to know for your exams
5 .
Which of the following is NOT true of many of the elements in this block of elements?

They form coloured compounds
They are extremely reactive
They are often made into alloys
Have a high melting and boiling point
Most of them do form compounds but not usually as vigorously as the reactive metals either side of this block of the periodic table
6 .
The picture shows neon lights. Which group of the periodic table does neon belong to?

1
2
6
8
Neon is a gas from group 8. It has 8 electrons in its outer shell and is therefore inert
7 .
The picture shows an early version of the periodic table. The elements are arranged by order of atomic weight. Whose periodic table is this?
Newlands
Dalton
Mendeleev
Rutherford
He realised that there were still more elements to be discovered and what makes his periodic table stand out from the others is that it had gaps so that these could be fitted in when they were found
8 .
Metals are found in which coloured areas of the periodic table in the diagram?
Yellow, orange, pale blue, dark blue
Yellow, pale blue, white, dark blue
Dark blue, red, yellow, white
White, dark blue, yellow, pale blue
Metals make up the majority of the elements and are found to the left side of the table
9 .
**Unlimited Quizzes Await You! 🚀**
Hey there, quiz champ! 🌟 You've already tackled today's free questions.
Ready for more?
Ready for more?
🔓 Unlock UNLIMITED Quizzes and challenge yourself every day. But that's
not all...
not all...
🔥 As a Subscriber you can join our thrilling "Daily Streak" against other
quizzers. Try to win a coveted spot on our Hall of Fame Page.
quizzers. Try to win a coveted spot on our Hall of Fame Page.
Don't miss out! Join us now and keep the fun rolling. 🎉
**Unlimited Quizzes Await You! 🚀**
Hey there, quiz champ! 🌟 You've already tackled today's free questions. Ready for more?
🔓 Unlock UNLIMITED Quizzes and challenge yourself every day. But that's not all...
🔥 As a Subscriber you can join our thrilling "Daily Streak" against other quizzers. Try to win a coveted spot on our Hall of Fame Page.
Don't miss out! Join us now and keep the fun rolling. 🎉